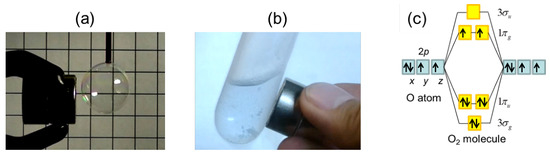
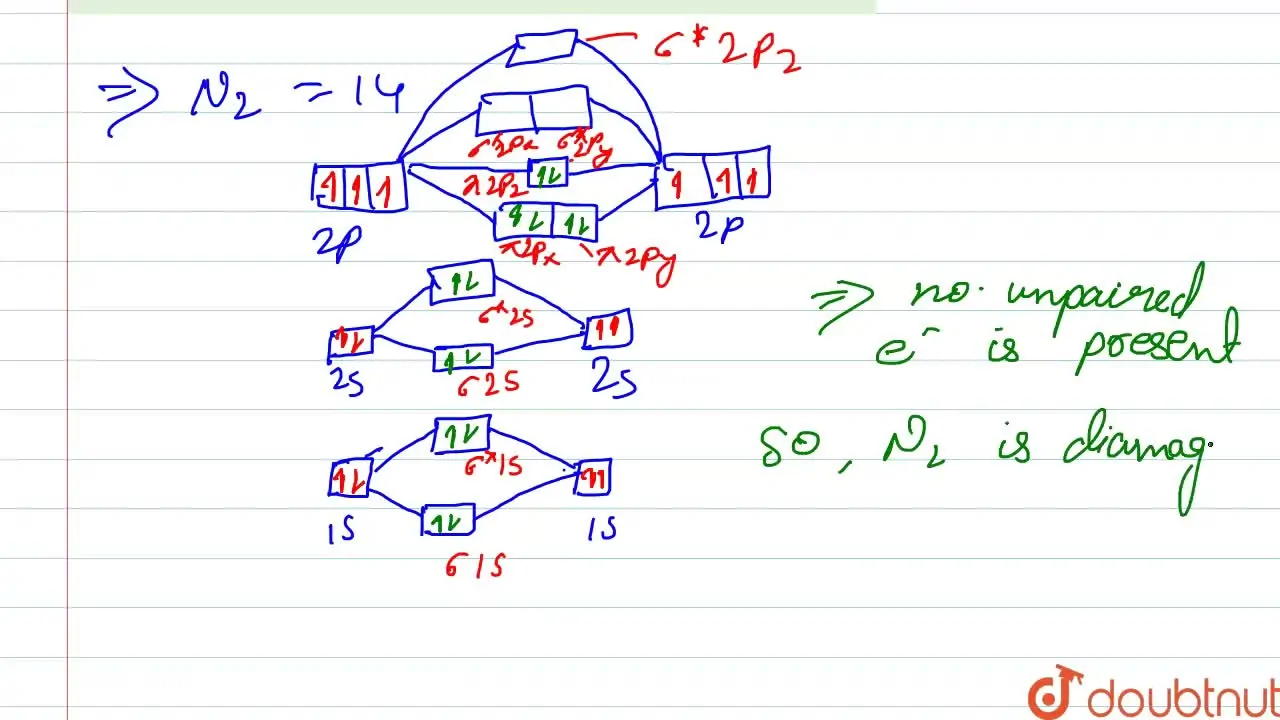
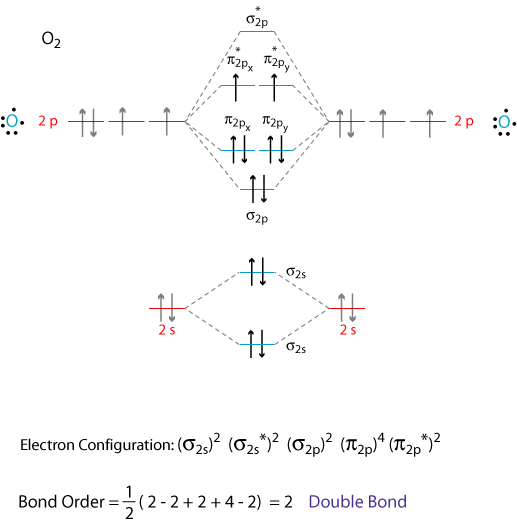
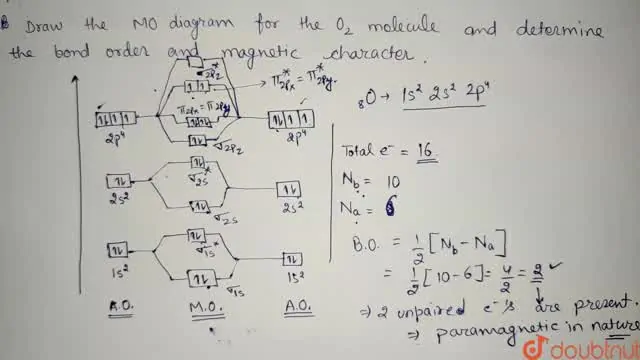
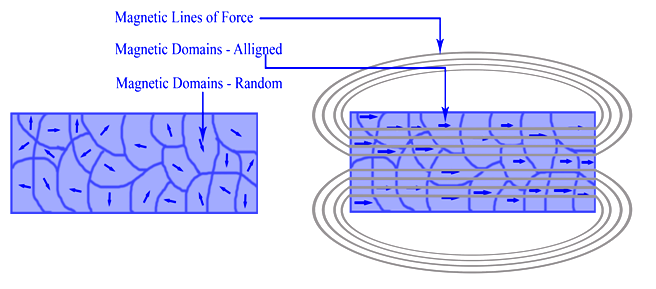
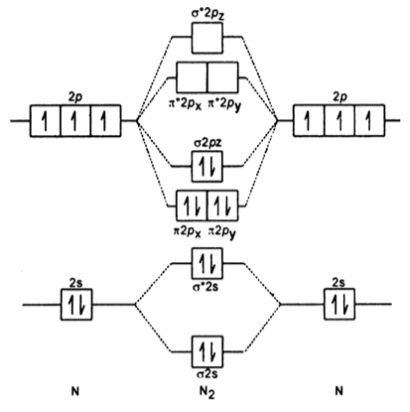
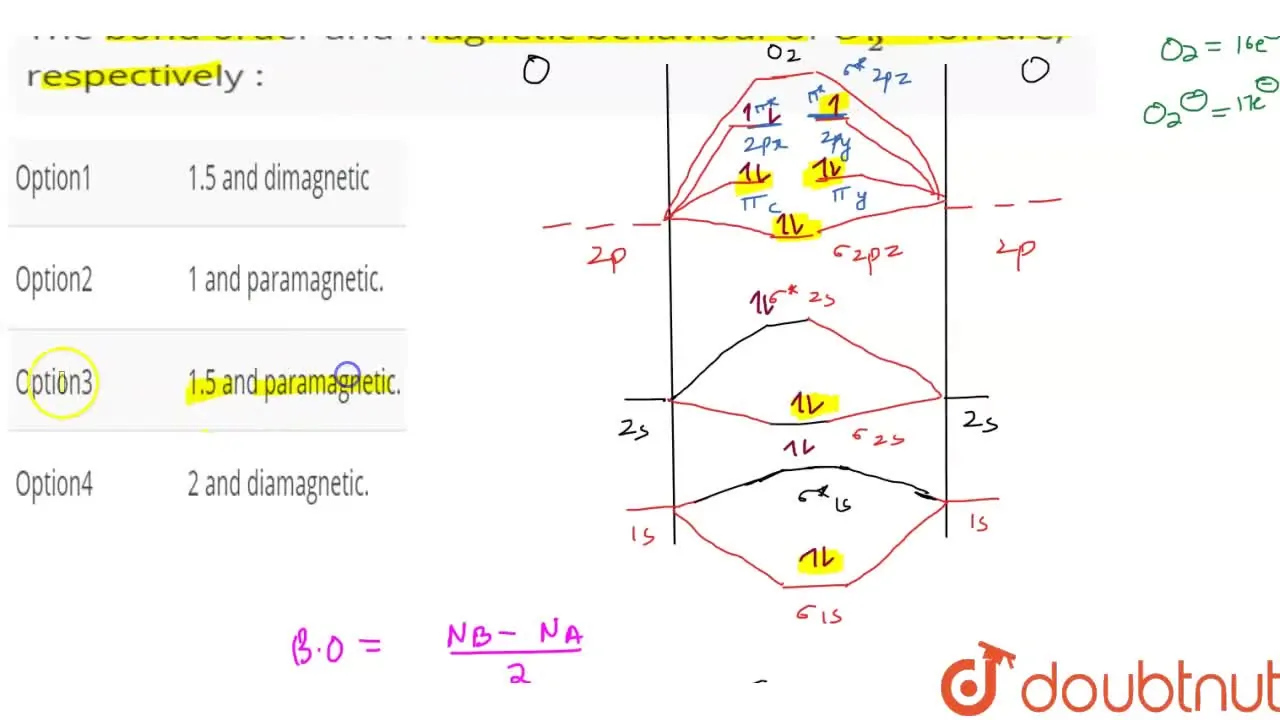
Molecular Orbital Theory || MOT || BMOs and ABMOs|| Bond Order || Magnetic Behaviour|| H2 Formation - YouTube

compare the relative stabilities of O2- and N2+and comment on their magnetic behaviour - Chemistry - Chemical Bonding and Molecular Structure - 13191415 | Meritnation.com

use molecular orbital theory to explain the magnetic behaviour and bond order of O2 Negative and O2+ molecules - Chemistry - Chemical Bonding and Molecular Structure - 13154833 | Meritnation.com